Translate this page into:
Measurement of Primary Stability of Mini Implants Using Resonance Frequency Analysis
This article was originally published by Wolters Kluwer and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction
In the last decade, anchorage control with mini-implants has gained enormous credibility in maintaining orthodontic anchorage. Resonance frequency analysis (RFA) has proven to be an adequate method to measure the stability of these mini-implants because of its non-invasiveness and contactless measurement method.
Materials and Method
Tomas and S.K surgical mini-implants were tested. For this purpose custom fabricated attachment was fabricated to attach the smart peg on orthodontic mini-implant head, and 45 mini-implants were inserted in fresh swine pelvic bone in the density matched sites to that of most common sites where mini-implants are placed in human mandible. Mini-implants of two different lengths with diameter constant were also placed to assess the effect of length on primary stability.
Results
The mean ISQ of Group 1 (Tomas 10 mm) was 55.53±3.39 while that of Group 2 (S.K Surgical 10mm) was 56.63±3.48 and that of Group 3(S.K Surgical 8 mm) was 55.90±3.48. Difference among the groups were not statistically significant when ANOVA test was used (P >0.05).
Conclusion
The resonance frequency analysis is applicable to comparatively assess the primary stability of orthodontic mini-implants. There was no difference in primary stability of Tomas and S.K Surgical mini-implant and primary stability was not affected by the length of the mini-implant.
Keywords
Mini implants
primary stability
resonance frequency analysis
Introduction
According to Graber,[1] orthodontic anchorage refers to the nature and degree of resistance to displacement offered by an anatomic unit when used for the purpose of moving teeth. Anchorage stability is a basic success factor in orthodontic treatment. That is why skeletal anchorage is established, especially in complex cases.
The clinical advantages of skeletal anchorage over dental and extraoral anchorage are absolute stability and independence from patient compliance. The basic requirement for the success of orthodontic mini implants is sufficient primary stability. Primary stability basically comes from mechanical interlocking with the cortical bone when the mini implant is placed. Primary stability is influenced by bone quality and quantity, surgical technique, and screw geometry.[2] Bone of soft quality with <0.5 mm of cortical thickness has been suggested to increase the risk of failure.[3]
Determining primary stability after insertion can help predict success of the orthodontic mini implant. The first methods used to clinically evaluate implant stability were the tapping method,[4] radiography,[5] and the Periotest.[6] However, all these methods lack enough precision and repeatability in quantifying stability; therefore, a precise and repeatable measure of implant stability was needed.[7]
Resonance frequency analysis (RFA) has proven to be an adequate method because of its noninvasiveness and contactless measurement method. RFA measurement requires a small magnet in an aluminum housing to be screwed on top of the implant head which is called a SmartPeg. Electromagnetic pulses with frequencies ranging from 5 to 15 kHz are emitted by a handpiece toward the SmartPeg. The device detects the resonance frequency of the SmartPeg implant unit in the bone. The unit of measurement is the implant stability quotient (ISQ) which ranges from 0 to 100. Higher the ISQ value higher is the stability of the implant. RFA is regarded as the gold standard for clinical stability measurement of dental implants.[8]
Hence, the aim of this study was to evaluate the primary stability of two different titanium mini implants using RFA.
Materials and Methods
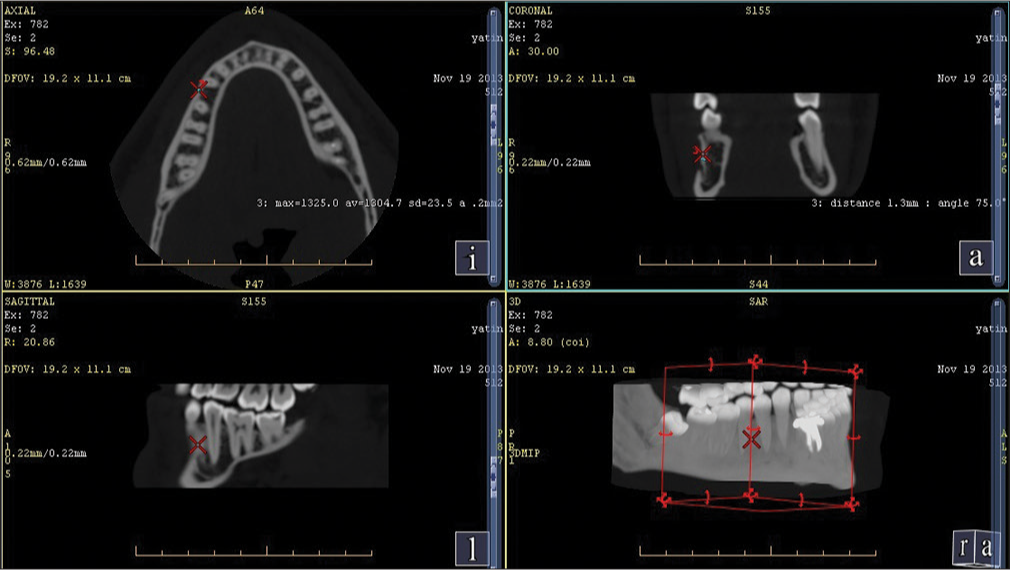
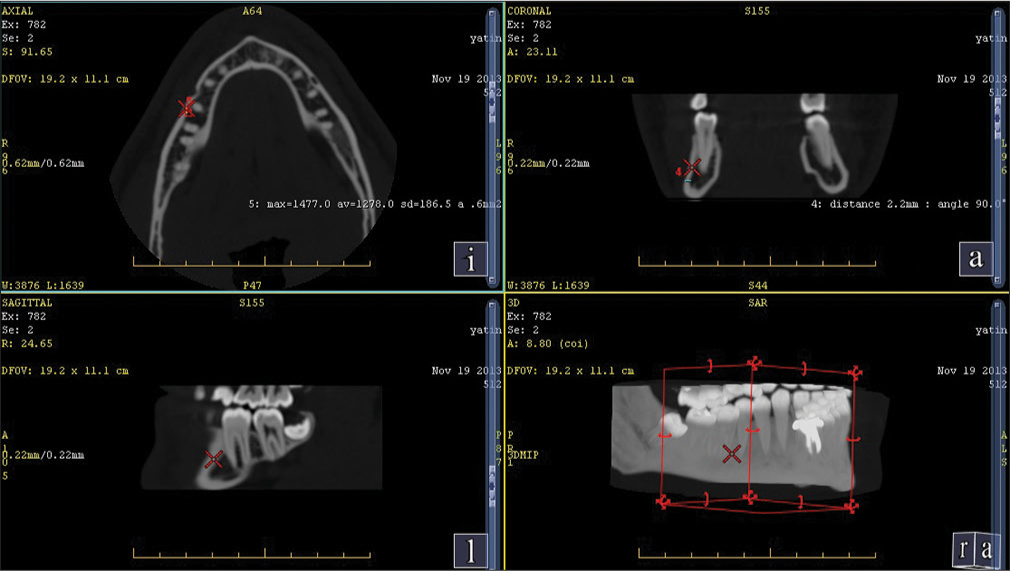
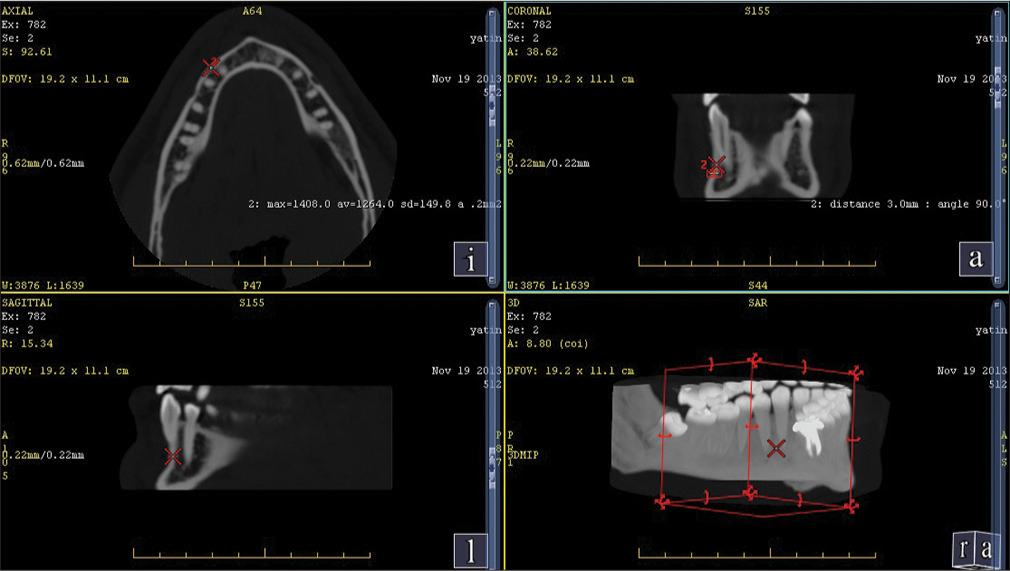
A spiral-computed tomography (CT) (G. E Optima 660) 384 slice scan of a human mandible was done to assess the bone density at the commonly used sites where the mini implants are placed.[9] The most common sites found were mainly in the interradicular region in the second and first molar, second and first premolar, first molar and second premolar at 11 mm from the alveolar crest, and first premolar and canine at 11 mm from the alveolar crest.
The average cortical bone density found in the human mandible is shown in Table 1.
| Region | Average cortical bone density (HU) | Misch classification |
|---|---|---|
| Interradicular region in the second and first molar [Figure 1] | 1472 | D1 |
| Second and the first premolar [Figure 2] | 1392 | D1 |
| First molar and second premolar at 11 mm from alveolar crest [Figure 3] | 1278 | D1 |
| First premolar and canine at 11 mm from alveolar crest [Figure 4] | 1264 | D1 |
HU – Hounsfield unit
Fresh swine pelvic bone was harvested, and a spiral-CT scan was done on the same day to assess the various sites of differing bone density, and then these sites were matched with human mandible scan of young adult to select sites for placement. The sites selected on the swine pelvic bone had an average cortical bone density of 1432 Hounsfield units (HU) (D1 according to Misch classification)[10] [Figure 5].

- Bone density in interradicular region in second and first molar
- Bone density between second and first premolar
- Bone density between first molar and second premolar at 11 mm from alveolar crest
- Bone density between first premolar and canine at 11 mm from alveolar crest
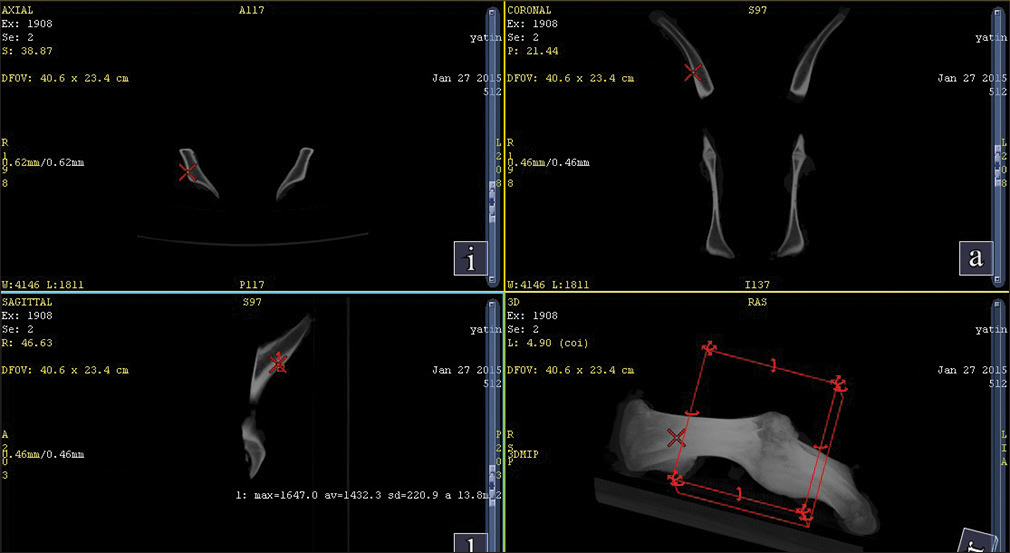
- Average cortical bone density of swine pelvic bone
The bone was split used into two halves from the midline and 15 implants (Group 1, Tomas 10 mm) were placed on the right side and 15 implants (Group 2, S. K Surgical 10 mm) were placed on the left side in the region of similar bone density as that of human mandible [Figure 6].

- Mini implants placed on contralateral side of similar bone density
The SmartPeg was first attached to the custom-fabricated attachment, and the connection was made finger tight as per the manufacturers guidelines as shown in Figure 7. After attaching the SmartPeg to the custom-fabricated attachment it was then attached to implant head, and the screw in the custom-fabricated attachment was tightened using the Allen key so that a firm connection is obtained between the SmartPeg and the implant [Figure 8]. Then, Osstell RFA device was held parallel to the bone, and primary stability was assessed for both the groups [Figure 9]. The assessment was done in two different directions perpendicular to each other but parallel to the bone, and the mean was calculated.

- Attachment of the SmartPeg to the custom-fabricated attachment
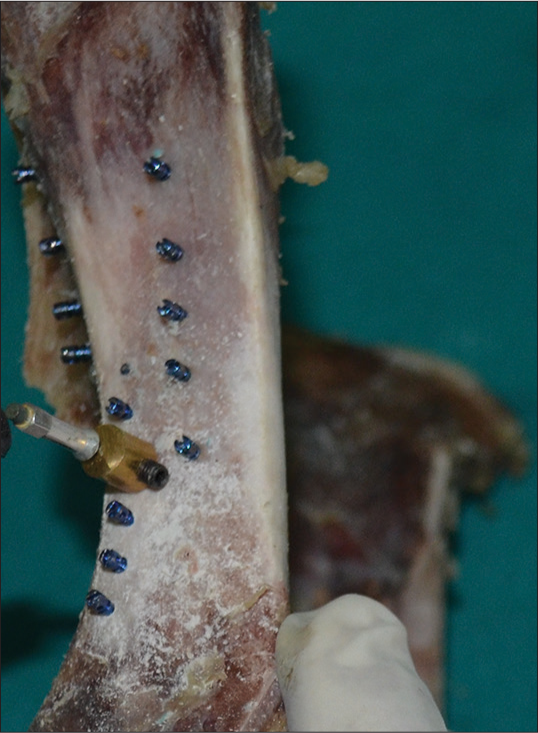
- Attachment of the smart peg to the implant head

- Primary stability measured using Osstell resonance frequency analysis device
Then, similar bone density sites were assessed again and 15 implants (Group 3 S. k surgical, 8 mm) were placed in the region of similar bone density as of Group 1 and Group 2 (Group 3 S. k surgical, 8 mm) implants were used to assess the effect of length if any on primary stability, and Osstell RFA device was used to assess their primary stability in the similar manner.
None of the implants got fractured while placement and all the implants where stable after placement.
Results
The data were arranged systematically, and information was transferred onto the master chart created in Microsoft Excel (2010) for the purpose of data analysis. The Statistical software, namely, SPSS (version 17.0) was used for the analysis of the data. Data comparison was done by applying one-way anova test to find out the statistical significance of the comparisons. Quantitative variables were compared using mean values. Significance level was fixed at P < 0.05.
The mean ISQ of Group 1 (Tomas 10 mm) was 55.53 ± 3.39 while that of Group 2 (S. K Surgical 10 mm) was 56.63 ± 3.48 and that of Group 3(S. K Surgical 8 mm) was 55.90 ± 3.48 [Graph 1]. There was no significance difference found in primary stability between three groups: Group 1 (Tomas 10 mm), Group 2 (S. K Surgical 10 mm), and Group 3 (S. K Surgical 8 mm). The P value calculated was 0.6 which is >0.05 [Table 2].

- Mean implant stability quotient of the three groups
| ANOVA | |||||
|---|---|---|---|---|---|
| Sum of squares | df | Mean squares | F | Significant | |
| Between groups | 9.411 | 2 | 4.706 | 0.395 | 0.676 |
| Within groups | 500.067 | 42 | 11.906 | ||
| Total | 509.478 | 44 | |||
Discussion
Anchorage control is an important factor in the success of orthodontic treatment. There have been many attempts to devise suitable anchorage methods including intraoral and extraoral appliances. The intraoral methods include a tooth or group of teeth, and extraoral appliance use headgear for anchorage in orthodontics. When extraoral devices are employed, anchorage can be stable; however, it depends on patient’s cooperation. Intraorally derived anchorage is unstable and may result in loss of anchorage. The only advantage of intraorally derived anchorage is that it does not require extensive cooperation from the patient.[11]
In the last decade, anchorage control with mini implants has gained enormous credibility in the clinical management of space closure. They are small, can be used in a variety of host sites, insertion procedure is less traumatic and they can be loaded soon after placement. The introduction of screws for orthodontic anchorage has greatly extended orthodontic treatment options. Despite their temporary clinical service, the failure rate of orthodontic mini implants has been reported to be higher than that of machined dental implants.[12,13] Dental implant placement usually involves a healing period, followed by achievement of secondary stability that is additional bone-implant contact from the osseointegration process. Therefore, the success of mini implants completely depends on their primary stability which comes from mechanical interlocking with the cortical bone when the mini implant is placed.
As mini implants have been incorporated in our routine orthodontic practice and they are loaded immediately after the placement, so to reduce the failure rate it is very important to assess their primary stability. The purpose of this study was to assess the primary stability of the mini implants in a noninvasive manner which can be used clinically in an efficient way.
RFA is regarded as the gold standard for clinical stability measurement of dental implants. One of the methods to check the primary and secondary stability of orthodontic mini implant is measuring the peak insertion and removal torque values.[14,15] Disadvantage with this method is that it can be done only during insertion and removal of the mini implant. Quantitative computerized tomography[16] or quantitative cone-beam CT may have a correlation to primary implant stability but radiation exposure is always a concern for patients. A percussion testing instrument may be a convenient tool to evaluate the stability of an orthodontic mini implant at different time points; however, the handheld instrument sometimes delivers controversial clinical results because of a variety of factors.[17] A study done by Nienkemper et al.[18] have found a strong correlation between Periotest values and RFA and they have suggested that RFA is a feasible measurement method for orthodontic mini implants.
Therefore, it would be desirable to use RFA to evaluate orthodontic mini implants as well. One difficulty is the connection between the mini implant and the SmartPeg. Because of the sensitivity of this measurement technique, a stable, solid, and reproducible connection must be ensured to make it work. Most dental implant systems have an inner screw thread so the RFA system is based on a screw coupling. However, this screw coupling cannot be made on the mini implant head as the head of the mini implant is used for various mechanics purpose, and moreover, the head of the mini implant is small to provide a screw coupling.
The first pilot study (2009)[19] regarding RFA for mini implants used adhesive fixation of a magnet to the mini implant’s head. The results suggested that RFA might work for mini implants. However, it should be noted that the bonding strength between the transducer and the implant could be affected by factors such as acrylic resin thickness, moisture contamination, or available bonding area. Moreover, it is still unknown whether similar results could be obtained with different adhesives. Veltri et al.[20] soldered the SmartPeg over the implant head using an abutment. However, this method was not suitable as the soldering process might affect the magnetism property of the SmartPeg. Nienkemper et al.18] modified the head of the mini implant to provide a direct attachment of the SmartPeg. The mini implants used in this had industrially fabricated inner thread at the top. It was made for fixation of prefabricated abutments to create different kind of mechanics. In cooperation with Osstell, the SmartPeg type 1 was modified. However, this was not clinically possible as the modification of the implants was done industrially and the mini implants have to be sent to Osstell to for such fabrication.
To carry out the RFA with the Osstell instrument, custom-fabricated attachment was needed to connect the SmartPeg to the mini implant. This modification was believed not to affect measurement accuracy based on the following considerations. The resonance frequency of dental implants is influenced by the orientation of the transducer,[21] the bone transducer distance,[22,23] and the overall stiffness of the system, including the transducer-implant and bone-implant interfaces.[22,23] Under the conditions of this study, the transducer had a standardized orientation and was screwed finger tight to the attachment according to the manufacturer’s instructions. Custom-fabricated attachment altered the bone transducer distance, but care was taken, and the height of the attachment for both the mini implant groups was kept same. Finally, screw locking of the attachment on the implant head provided a firm connection between the implant and the SmartPeg. Thus, the only parameter with the possible influence on the RFA measurement was the stiffness of the bone screw interface.
The advantages of the mode of attachment used in the present study were that neither the implant head needs to be modified and the attachment can easily be connected on the implant head, so it can be used in clinical situations very efficiently.
There are many studies confirming the importance of the contact between implant and surrounding bone.[24,25] In particular, the contact with the cortical bone seems to be important for implant stability. A correlation could be found between the bone to implant contact rate and RFA. Hence, to make a more clinically relevant study, cortical bone density of the most common sites where mini implants are placed as suggested by Poggio et al.[9] Of a young human mandible was measured and then similar bone density was found in pelvic bone of pig to select the sites for placement of mini implants. Pelvic bone of pig[19] was chosen for the study because it was found that the cortical bone density of pig was more comparable with that of young human mandible and similar bone density sites could be found in the bone.
Clinicians have been diagnosing, treatment planning, placing, and restoring dental implants using periapical and panoramic radiographs to assess bone anatomy for several decades. Two-dimensional images have been found to have limitations because of inherent distortion factors and the noninteractive nature of film. With the advent of technology, CT has led to a new era of implant imaging. The individual element of the CT image is called a voxel, which has a value, referred to in HU that describes the density of the CT image at that point. HU also known CT numbers, range from-1000 (air) to + 3000 (enamel), each corresponding to a different level of beam attenuation (Resnik et al., 2008).[26,27] The density of structures within the image is absolute and quantitative and can be used to differentiate tissues in the region (i.e., muscle, 35–70 HU; fibrous tissue, 60–90 HU, cartilage, 80–130 HU; bone 150–1800 HU) and characterize bone quality (D1 bone, >1250 HU; D2 bone, 750–1250 HU; D3 bone, 375–750 HU; D4 bone, <375 HU).[10]
The utility of CT for dental implant treatment planning was evident; however, the access to these imaging techniques is limited. Nevertheless, CT scans are not without their limitations/concerns and radiation exposure and cost are the major two.[26,28,29]
The result of this study is in accordance with the study done by Pan et al.[30] in which they compared difference in primary stability between titanium and stainless steel mini implants and concluded that there was statistically no significant difference found in their primary stabilities. They also concluded that despite the numerous differences between stainless steel and titanium alloy, both materials offer similar success in fulfilling the main mechanical requirements of stability in mini implants.
In the experimental design by Wilmes et al.,[31] it was found that the length of the mini implant does not have significant effects on their stability when measuring insertion torque.
Chen et al.[32] did a systemic review on critical factors for the success of orthodontic mini implants and stated that primary stability is not affected by the length of mini implant.
Holm et al.[33] did an in vitro study on factors affecting the primary stability of orthodontic mini implants. They stated that mean insertion torque was more affected by changes in external diameter than because of the length.
In contrast to the above studies, Tseng et al.[34] found the length of the inserted mini implant to be an important risk factor.
The result of the present study also shows that there was no significant difference between the primary stability when the length of the implant is altered (P = 0.568).
A study done by Miyamoto et al. (2005) stated that dental implant stability at the time of surgery was weakly influenced by implant length, but cortical bone thickness strongly increases implant stability.
The result of the present study is in accordance with an experimental study done by Nimmi et al. (1997) demonstrating that removal torque for implants in the fibula, iliac crest, and scapula of cadavers was related to cortical bone thickness, not total bone thickness.
Implant stability depends largely on cortical bone thickness, and therefore, the application of longer implants is not effective to increase primary implant stability as the surface area of the mini implant remaining in the cortical bone is same for different length of mini implant.
Like every other study, this study has its own limitations. Because of the unavailability of transducers adaptable to orthodontic mini implants, a custom-fabricated attachment was made to connect the SmartPeg to the implant head so the chances of errors might have increased. Second, as only primary stability was evaluated, so there are chances that implant length might be affected by healing process between the implant surface and the bone, i.e., secondary stability. Further clinical investigations must be performed using RFA to see the stability related changes during healing.
The use of the RFA technology deserves attention for its potential to conservatively provide repeatable and comparable measurements of orthodontic screw retention.
Conclusion
The result of the present study suggests that RFA can be used as measurement method for mini implant stability
There was no difference in primary stability of Tomas and S. K Surgical mini implant
Primary stability was not affected by the length of the mini implant
In the future, RFA can be used routinely to assess mini implant stability throughout the course of treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Orthodontics – Principles and Practice. Philadelphia, PA: W.B. Saunders; 1961.
- A comparison of two methods of enhancing implant primary stability. Clin Implant Dent Relat Res. 2004;6:48-57.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical proceures In: Brånemark PI, Zarb G, Albrektsson T, eds. Osseointegration in Clinical Dentistry. Chicago: Quintessence; 1985. p. :232.
- [Google Scholar]
- Crestal bone changes around titanium implants: A methodologic study comparing linear radiographic with histometric measurements. Int J Oral Maxillofac Implants. 2001;16:475-85.
- [CrossRef] [Google Scholar]
- Periotest – A dynamic procedure for the diagnosis of the human periodontium. Clin Phys Physiol Meas. 1990;11:65-75.
- [Google Scholar]
- Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998;11:491-501.
- [CrossRef] [PubMed] [Google Scholar]
- Resonance frequency analysis and damping capacity assessment. Part 2: Peri-implant bone loss follow-up. An in vitro study with the periotest and osstell instruments. Clin Oral Implants Res. 2006;17:80-4.
- [CrossRef] [Google Scholar]
- “Safe zones”: A guide for miniscrew positioning in the maxillary and mandibular arch. Angle Orthod. 2006;76:191-7.
- [Google Scholar]
- Density of bone: Effects on surgical approach and healing In: Contemporary Implant Dentistry. Canada: Mosby, Elsevier; 2008. p. :645-67.
- [Google Scholar]
- Studies on the efficacy of implants as orthodontic anchorage. Am J Orthod. 1983;83:311-7.
- [Google Scholar]
- A prospective study of the risk factors associated with failure of mini-implants used for orthodontic anchorage. Int J Oral Maxillofac Implants. 2004;19:100-6.
- [Google Scholar]
- Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur J Oral Sci. 1998;106:527-51.
- [Google Scholar]
- Biomechanical and histological comparison of self-drilling and self-tapping orthodontic microimplants in dogs. Am J Orthod Dentofacial Orthop. 2008;133:44-50.
- [Google Scholar]
- Removal torque of miniscrews used for orthodontic anchorage – A preliminary report. Int J Oral Maxillofac Implants. 2006;21:283-9.
- [Google Scholar]
- Bone density assessments of dental implant sites: 2. Quantitative cone-beam computerized tomography. Int J Oral Maxillofac Implants. 2005;20:416-24.
- [CrossRef] [Google Scholar]
- Validity and clinical significance of biomechanical testing of implant/bone interface. Clin Oral Implants Res. 2006;17(2):2-7.
- [Google Scholar]
- Measurement of mini-implant stability using resonance frequency analysis. Angle Orthod. 2013;83:230-8.
- [Google Scholar]
- Application of a wireless resonance frequency transducer to assess primary stability of orthodontic mini-implants: An in vitro study in pig ilia. Int J Oral Maxillofac Implants. 2009;24:647-54.
- [Google Scholar]
- Soft bone primary stability of 3 different miniscrews for orthodontic anchorage: A resonance frequency investigation. Am J Orthod Dentofacial Orthop. 2009;135:642-8.
- [Google Scholar]
- Influence of transducer orientation on osstell stability measurements of osseointegrated implants. Clin Implant Dent Relat Res. 2007;9:60-4.
- [Google Scholar]
- Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin Oral Implants Res. 1996;7:261-7.
- [CrossRef] [PubMed] [Google Scholar]
- Resonance frequency measurements of implant stability in vivo. A cross-sectional and longitudinal study of resonance frequency measurements on implants in the edentulous and partially dentate maxilla. Clin Oral Implants Res. 1997;8:226-33.
- [CrossRef] [PubMed] [Google Scholar]
- Biological and biomechanical evaluation of bone remodelling and implant stability after using an osteotome technique. Clin Oral Implants Res. 2005;16:1-8.
- [Google Scholar]
- Interface reaction at dental implants inserted in condensed bone. Clin Oral Implants Res. 2005;16:509-17.
- [CrossRef] [PubMed] [Google Scholar]
- Dental implants In: White SC, Pharoah MJ, eds. Oral Radiology Principles and Interpretation. St. Louis, Missouri: Mosby, Elsevier; 2009. p. :597-612.
- [Google Scholar]
- Advanced imaging In: White SC, Pharoah MJ, eds. Oral Radiology Principles and Interpretation. St. Louis, Missouri: Mosby, Elsevier; 2009. p. :207-24.
- [Google Scholar]
- Dental imaging in implant treatment planning. Implant Dent. 2010;19:288-98.
- [CrossRef] [PubMed] [Google Scholar]
- What is cone-beam CT and how does it work? Dent Clin N Am. 2008;52:707-30.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of different implant materials on the primary stability of orthodontic mini-implants. Kaohsiung J Med Sci. 2012;28:673-8.
- [Google Scholar]
- Parameters affecting primary stability of orthodontic mini-implants. J Orofac Orthop. 2006;67:162-74.
- [CrossRef] [PubMed] [Google Scholar]
- Critical factors for the success of orthodontic mini-implants: A systematic review. Am J Orthod Dentofacial Orthop. 2009;135:284-91.
- [Google Scholar]
- An in vitro study of factors affecting the primary stability of orthodontic mini-implants. Angle Orthod. 2012;82:1022-8.
- [CrossRef] [PubMed] [Google Scholar]
- The application of mini-implants for orthodontic anchorage. Int J Oral Maxilliofac Surg. 2006;35:704-7.
- [CrossRef] [Google Scholar]






